Molality, Molarity and Mole Fraction
Molality, also called molal concentration, is a measure of the concentration of a solute in a solution in terms of amount of substance in a specified amount of mass of the solvent. This contrasts with the definition of molarity which is based on a specified volume of solution.
The properties and behavior of many solutions depend not only on the nature of the solute and solvent but also on the concentration of the solute in the solution. Chemists use many different units when expressing concentration; however, one of the most common units is molarity. Molarity (M) is the concentration of a solution expressed as the number of moles of solute per liter of solution.
Mole fraction is another way of expressing the concentration of a solution or mixture. It is equal to the moles of one component divided by the total moles in the solution or mixture.
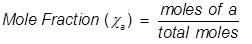
a = the component that is being identified for mole fraction
Mole fraction is used in a variety of calculations, but most notably for calculating partial pressures.



Comments
Post a Comment